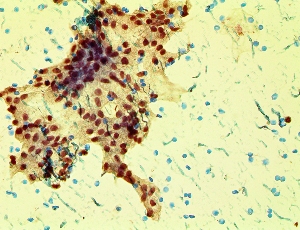
Better Diagnostic Information = Better Patient Management
The proprietary WaveSense targeted cell isolation and enrichment technology transforms non-invasive liquid biopsies into valuable data sources that enable enhanced diagnostic information.

Like Finding A Needle In A Haystack
Our Prost8 test ...
Differentiates prostate cancer from BPH
Identifies false-negative biopsy results
Monitors your patients